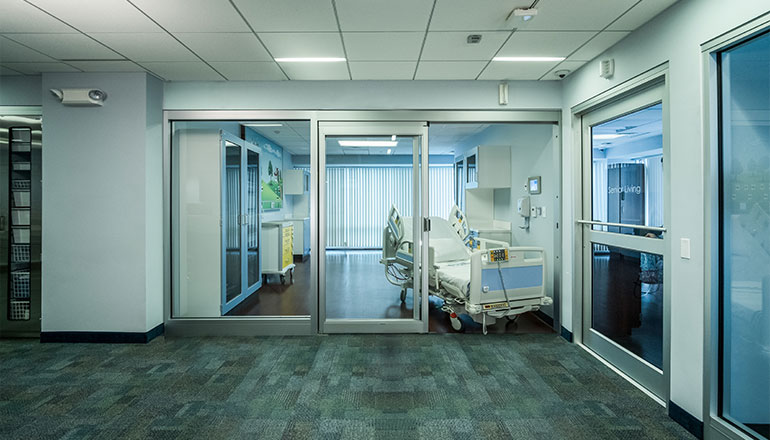
Privacy Glazing Solution That Performs
Delivering precision control and seamless integration, our advanced privacy glazing solutions are engineered for the demands of modern spaces. Featuring operable or fixed louvers fully enclosed within a hermetically sealed glass unit, they offer a clean, maintenance-free way to manage privacy, daylight, and solar heat gain—without the need for strings or cords.
Whether for health care, commercial, educational, or institutional settings, these systems allow for total design flexibility and can be tailored to fit virtually any configuration. From patient rooms to meeting areas, they offer a superior approach to privacy that meets the highest performance standards.
Optimized for:
- Infection prevention and easy cleaning
- On-demand privacy and visibility control
- Enhanced daylighting
- Solar heat gain management
 Featured ProductSchlage Credential Services deliver control, security and flexibility without limiting your options. From encryption keys to card tracking and custom artwork, our services help create an access control plan that fits today and grows with you tomorrow.
Featured ProductSchlage Credential Services deliver control, security and flexibility without limiting your options. From encryption keys to card tracking and custom artwork, our services help create an access control plan that fits today and grows with you tomorrow.